HumanKine cGMP Cytokines and Growth Factors
The largest supplier of cGMP recombinant proteins, in stock and ready to ship.
Why Choose the Proteintech HumanKine™ cGMP Recombinant Proteins?
Applicable Solutions

Related Documentation
FAQ
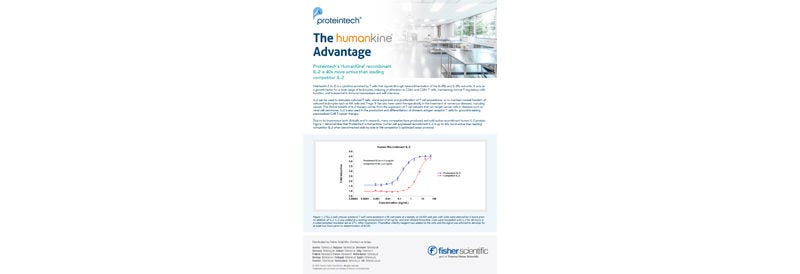
GMP proteins come with extensive documentation for traceability, as well as additional quality control testing and quality assurance reviews, whereas the RUO grade line offers reliable products which are more cost-effective during early research and development. As the manufacturing process is the same for RUO and GMP products, HumanKine offers a seamless preclinical-to-clinical transition of product lines, saving a significant amount of time and money.
Unless specified otherwise in the product information sheet, our products are formulated to ensure the stability of lyophilized proteins at room temperature. It is advised to store lyophilized products at -20°C to -80°C. For reconstituted solutions of most products, short-term storage at 4°C is recommended. Extended storage of the protein solution should be at -20°C to -80°C. Keep in mind that each freeze/thaw cycle may lead to some protein denaturation, so it is not recommended to subject aliquots to multiple freeze/thaw cycles.
The US-FDA does not audit or certify manufacturing facilities that produce ancillary materials. HumanKine GMP products are manufactured under the ISO13485 quality management system adhering to USP and European pharmacopeia recommendations to ensure potency, purity, and safety. The manufacturing facility can be audited by the end user upon request.

Reliable cGMP Tools
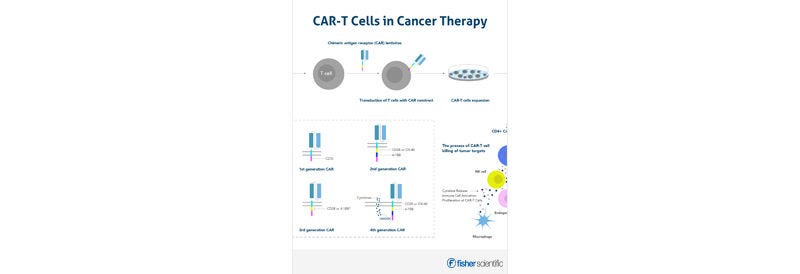
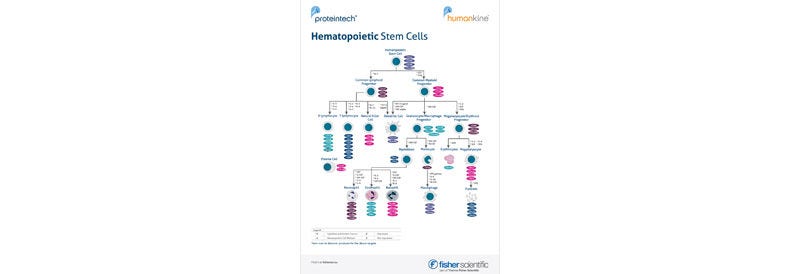
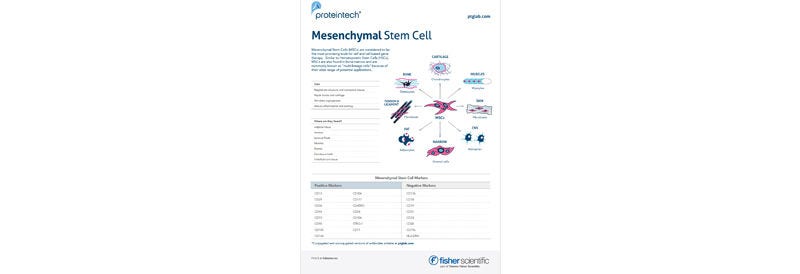
Browse our selection of Humankine cGMP-grade cytokines and growth factors from Proteintech — produced in a human expression system to ensure superior bioactivity and consistency. Ideal for clinical and cell therapy applications. Explore the range and accelerate your research today.